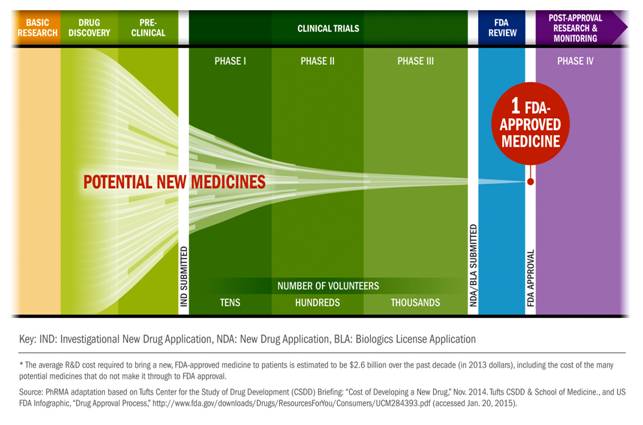
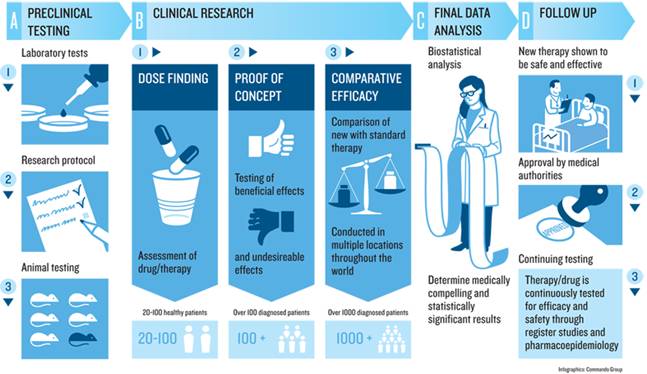
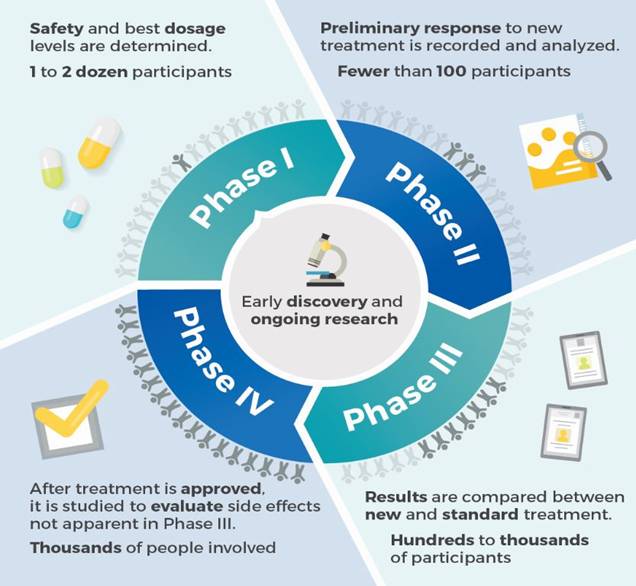
Qua nhiều hội nghị, hội thảo quốc tế và trong nước ở nhiều chuyên ngành khác nhau chúng , phần lớn các tác giả công trình nghiên cứu đã áp dụng các biểu mẫu đánh giá theo đề cương quy định của thử nghiệm lâm sàng (clinical trials) của quy định CITI, FDA và tiêu chuẩn châu Âu (European Agency for the Evaluation of Medicinal Products_EMEA), hoặc Nhật Bản. Do vậy, các nghiên cứu thử nghiệm thuốc hoặc các thuốc, công cụ, dụng cụ y khoa (drugs, medical devices) trở nên chặt chẽ và có giá trị khoa học, đồng thời giúp cho các nhà lâm sàng, tiếp cận và làm các thử nghiệm như thế đúng quy trình và không vi phạm đạo đức tròn nghiên cứu.
Nhân đây, chúng tôi xin giới thiệu đến quý bạn đồng nghiệp các biểu mẫu chính thống có thể áp dụng tại nhiều quốc gia khác nhau về các lĩnh vực thử nghiệm kahcs nhau, chuyên ngành khác nhau để công trình nghiên cứu khoa học đúng phương pháp và đầy đủ hồ sơ. Anh (chị) có thể tham khảo thêm trang website: https://www.tga.gov.au/clinical-efficacy-and-safety-guidelines/ Clinical efficacy and safety guidelines (update 24Jan. 2017).
Để tìm kiếm các hướng dẫn bằng các từ khóa trên trang, sử dụng chức năng browser's 'Find' (Control-F or Edit, Find in most browsers). Một số chuyên mục khác có thể bạn đang quan tâm cũng có trên trang website này: Clinical pharmacology and pharmacokinetics | Alimentary tract and metabolism | Blood and blood-forming organs | Blood products (including biotech alternatives) | Cardiovascular system | Dermatologicals | Genito-urinary system and sex hormones | Anti-infectives for systemic use | Antineoplastic and immunomodulating agents | Rheumatology / musculoskeletal system | Nervous system | Respiratory system | Biostatistics | General | Radiopharmaceuticals and Diagnostic Agents | Allergy/Immunology
1. Clinical pharmacology and pharmacokinetics
*Large file warning: Attempting to open large pdf files over the Internet within the browser window may cause problems. It is strongly recommended you download this document to your own computer and open from there.
EMA/CHMP/37646/2009 (pdf,166kb) (link is external)
Guideline on the use of pharmacogenetic methodologies in the pharmacokinetic evaluation of medicinal products
Effective: 15 September 2014
EMA/CHMP/EWP/280/96 (pdf,499kb) (link is external)
Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms (EMA/CPMP/EWP/280/96 Corr1)
Replaces: CPMP/EWP/280/96 Corr. Note for Guidance on Modified Release Oral and Transdermal dosage forms: Section II (Pharmacokinetic and Clinical Evaluation) (corrected version adopted by TGA 1 June 2014)
Effective: 1 June 2015
TGA annotation: For multiple strengths of generic TDDS products, bioequivalence studies should be performed at least on the lowest and highest strengths versus the corresponding innovator products. If an applicant considers that this is unnecessary in a particular case, a justification for not submitting bioequivalence data should be submitted in accordance with the ARGPM guidance on Biopharmaceutic studies.
CPMP/EWP/560/95/Rev. 1 Corr.* (pdf,833kb) (link is external)*
Guideline on the Investigation of Drug Interactions
Replaces: CPMP/EWP/560/95 (Adopted by TGA 19 April 2001), and EMEA/CHMP/EWP/297931/2008 Concept Paper on this topic (provided for information 10 February 2009)
Effective: 1 August 2014
CPMP/EWP/QWP/1401/98 Rev. 1/ Corr ** (pdf,233kb) (link is external)
Guideline on the Investigation of Bioequivalence
Replaces: CPMP/QWP/EWP/1401/98 (Adopted by TGA 12 February 2002)
Effective: 16 June 2011
Hình 1
TGA annotation: While this guidance suggests that the design and conduct of the study should follow EU regulations on Good Clinical Practice, sponsors should note that the EU Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95) has been adopted in Australia with TGA annotations. The procedure for abridged applications claiming essential similarity to a reference product (i.e., generics), which allows applications to be made to numerous Member States of the EU, based on bioequivalence with a reference product from one Member State, does not apply in Australia. An application for registration of a generic product in Australia should generally include a bioequivalence study versus a leading brand obtained in Australia.
EMA/CHMP/600958/2010/Corr.* (pdf,138kb) (link is external)
Appendix IV of the Guideline on the Investigation on Bioequivalence (CPMP/EWP/QWP/1401/98 Rev.1): Presentation of Biopharmaceutical and Bioanalytical Data in Module 2.7.1
TGA annotation:
1.The procedure for abridged applications claiming essential similarity to a reference product (i.e., for generic medicines), which allows applications to be made to numerous Member States of the EU, based on bioequivalence with a reference product from one Member State, does not apply in Australia. Unless otherwise justified, an application for registration of a generic medicine in Australia should generally include one or more bioequivalence studies, each versus the Australian reference product. Directions given in this guideline regarding "non-EU reference products" (Section 2), and "Member State where the reference product is purchased from" (Table 2.1) should be disregarded in favour of the advice given in TGA Guidance 15: Biopharmaceutic studies.
2.Similarly, details of the (presumably most recent) EU Authority Inspection of the clinical and bioanalytical study sites and site of the PK and statistical analysis (Table 2.2) are GCP/GLP issues, and need not be provided.
3.Table 3.1 should be amended according to the metrics that are suitable for the product and/or study type.
Hình 2
2. Product-specific bioequivalence guidance
The European Medicines Agency's (EMA) has published a series of finalised product-specific bioequivalence guidelines which summarise in a standardised format the relevant study design principles for the demonstration of bioequivalence for specific products.
Unless specified otherwise, the TGA accepts these product specific requirements for bioequivalence demonstration described in finalised guidelines on this page:
The TGA effective date of the individual guidance is the effective date of the individual guidance.
For those products which comply with the current non-product-specific bioequivalence guidance (CPMP/EWP/QWP/1401/98 Rev. 1/ Corr) but not the product-specific guidance and for which an application to TGA is submitted within 2 years of the date of publication of the guidance, sponsors must provide a robust justification for not complying with the product-specific bioequivalence guideline.
EMA/618604/2008 (pdf,479kb) (link is external)
Questions & Answers: Positions on specific questions addressed to the pharmacokinetics working party
For information: 1 June 2014
EMEA/CHMP/EWP/192217/2009 (pdf,154kb) (link is external)
Guideline on bioanalytical method validation
Effective: 1 June 2013
CHMP/EWP/89249/2004 (pdf,98kb) (link is external)
Guideline on the Clinical Investigation of the Pharmacokinetics of Therapeutic Proteins
Effective: 6 January 2009
EMEA/CHMP/EWP/147013/2004 Corr (pdf,114kb) (link is external)
Guideline on the role of Pharmacokinetics in the Development of Medicinal Products in the Paediatric Population
Effective: 24 August 2009

Hình 3
EMEA/CHMP/SWP/28367/07 (pdf,83kb) (link is external)
Guideline on Strategies to Identify and Mitigate Risks for First-In-Human Clinical Trials with Investigational Medicinal Products
Effective: 26 June 2009
CHMP/EWP/185990/06 (pdf,68kb) (link is external)
Guideline on Reporting the results of Population Pharmacokinetic Analysis
Effective: 27 January 2009
CPMP/EWP/2339/02 (pdf,92kb) (link is external)
Guideline on the Evaluation of the Pharmacokinetics of Medicinal Products in Patients with Impaired Hepatic Function
Effective: 4 January 2006
EMA/83874/2014 (pdf,221kb) (link is external)
Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with decreased renal function
Replaces: CHMP/EWP/225/02 Note for guidance on the evaluation of the Pharmacokinetics of medicinal products in patients with impaired renal function (Adopted by TGA 24 November 2004)
Effective: 10 November 2016
CPMP/EWP/1875/03/Final (pdf,149kb) (link is external)
Points to Consider on the Clinical Requirements of Modified Release Products Submitted as a Line Extension of an Existing Marketing Authorisation
Effective: 7 June 2005
EMA/CHMP/594085/2015 (pdf,199kb) (link is external)
Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antimicrobial medicinal products
Replaces: CPMP/EWP/2655/99 Points to Consider on Pharmacokinetics and Pharmacodynamics in the Development of Antibacterial Medicinal Products(Adopted by TGA 19 April 2001)
Effective: 1 February 2017
3CC3A (pdf,39kb) (link is external)
Pharmacokinetic Studies in Man
Effective: 12 February 2002
3. Alimentary tract and metabolism
How to access a pdf document
EMA/CHMP/336243/2013 (pdf,219kb) (link is external)
Guideline on the evaluation of medicinal products for the treatment of chronic constipation (including opioid induced constipation) and for bowel cleansing
In place of: EMA/CHMP/462198/2012 Concept paper on the need of a guideline for clinical investigation of medicinal products for the treatment of chronic constipation (provided for information 1 August 2014)
Effective: 2 February 2016
CPMP/EWP/1080/00 Rev. 1 (pdf,225kb) (link is external)
Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus
Replaces: CPMP/EWP/1080/00 (Adopted by TGA 23 October 2002)
Effective: 1 April 2014
Hình 4
EMA/CHMP/EWP/342691/2009 (pdf,464kb) (link is external)
Guideline on the evaluation of drugs for the treatment of Gastro-oesophageal reflux disease
Effective: 15 September 2014
CPMP/EWP/2284/99 Rev. 1 (pdf,61kb) (link is external)
Guideline on the Development of New Medicinal Products for the Treatment of Crohn's Disease
Replaces: CPMP/EWP/2284/99 (Adopted by TGA 10 January 2002)
Effective: 25 February 2009
CHMP/EWP/18463/2006 (pdf,115kb) (link is external)
Guideline on the Development of New Medicinal Products for the Treatment of Ulcerative Colitis
Effective: 8 April 2009
EMA/CHMP/311805/2014 (pdf,164kb) (link is external)
Guideline on clinical evaluation of medicinal products used in weight management
Replaces: CPMP/EWP/281/96 Rev. 1 Guideline on Clinical Evaluation of Medicinal Products Used in Weight Control (Adopted by TGA 26 March 2010)
Effective: 6 January 2017
TGA annotation: If a sponsor wishes to claim secondary benefits associated with weight loss (such as improved blood lipids, glycaemic control or blood pressure), then the relevant guidelines for these specific benefits should be independently satisfied.
Hình 5
EMEA/CHMP/EWP/517497/2007 (pdf,51kb) (link is external)
Guideline on Clinical Evaluation of Medicinal Products Used in Weight Control (CPMP/EWP/281/96 Rev 1)
Addendum on Weight Control in Children
Effective: 25 February 2009
CPMP/EWP/4937/03 (pdf,112kb) (link is external)
Guideline on Non-Clinical and Clinical Development of Medical Products for the Prevention of Nausea and Vomiting Associated with Cancer Chemotherapy
Effective: 16 March 2009
CPMP/EWP/785/97 Rev. 1 (pdf,231kb) (link is external)
Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome
Replaces: CPMP/EWP/785/97 Points to consider on the evaluation of medicinal products for the treatment of Irritable Bowel Syndrome (adopted by TGA September 2004).
Effective: 25 May 2015
CPMP/EWP/863/98 (pdf,42kb) (link is external)
Points To Consider on Wording of Helicobacter Pylori Eradication Therapy in Selected SPC Sections
Effective: 15 December 2000
4. Blood and blood-forming organs
How to access a pdf document
CPMP/EWP/197/99 (pdf,44kb) (link is external)
Points to Consider Concerning Endpoints in Clinical Studies with Haematopoeitic Growth Factors for Mobilisation of Autologous Stem Cells
Effective: 19 April 2001
5. Blood products (including biotech alternatives)
How to access a pdf document
CHMP/BPWP/585257/2009 (pdf,147kb) (link is external)
Guideline on the clinical investigation of hepatitis B immunoglobulins
Effective: 2 February 2016
CHMP/BPWP/410415/2011 Rev 1 (pdf,246kb) (link is external)
Guideline on the clinical investigation of human normal immunoglobulin for subcutaneous and/or intramuscular administration (SCIg/IMIg)
Replaces: EMEA/CPMP/BPWG/283/00 Note for Guidance on the Clinical Investigation of Human Normal Immunoglobulin for Subcutaneous and Intramuscular Use (Adopted by TGA 12 March 2003)
Effective: 2 February 2016

Hình 6
CPMP/BPWG/575/99 Rev. 1 (pdf,115kb) (link is external)
Guideline on the Clinical Investigation of Human Anti-D Immunoglobulin for Intravenous and/or Intramuscular Use
Replaces: CPMP/BPWG/575/99 (Adopted by TGA 19 April 2001)
Effective: 10 February 2009
CPMP/BPWG/220/02 (pdf,142kb) (link is external)
Guideline on the Clinical Investigation of Human Plasma Derived Von Willebrand Factor Products
Effective: 1 June 2006
CPMP/BPWG/1089/00 (pdf,61kb) (link is external)
Guideline on the clinical investigation of Plasma derived Fibrin Sealant/Haemostatic Products
Effective: 13 January 2005
EMA/CHMP/BPWP/572810/2013 (pdf,125kb) (link is external)
Concept paper on the need for revision of the guideline on the clinical investigation of plasma derived fibrin sealant/haemostatic products (CPMP/BPWG/1089/00) and the related Core SmPC
For information: 1 August 2014
CPMP/BPWG/2220/99 (pdf,168kb) (link is external)
Note for Guidance on the Clinical Investigation of Plasma Derived Antithrombin Products
Effective: 16 October 2003
TGA annotation: If a sponsor believes that the patient numbers specified in this guideline are not achievable in Australia, advice should be sought from the TGA.
EMA/CHMP/BPWP/144533/2009 Rev 1. (pdf,332kb) (link is external)
Guideline on the clinical investigation of recombinant and human plasma-derived factor VIII products (Rev. 1)
Replaces: EMA/CHMP/BPWP/144533/2009 Guideline on the clinical investigation of recombinant and human plasma-derived factor VIII products (Adopted by TGA 1 June 2014)
Effective: 10 November 2016
EMA/CHMP/BPWP/144552/2009 Rev 1 (pdf,332kb) (link is external)
Clinical investigation of recombinant and Human plasma-derived factor IX products
Replaces: EMA/CHMP/BPWP/144552/2009
Guideline on clinical investigation of recombinant and human plasma-derived factor IX products (Adopted by TGA 1 June 2014)
Effective: 1 September 2015
EMA/CHMP/BPWP/94033/2007 rev. 2 (pdf,220kb) (link is external)
Guideline on the clinical investigation of human normal immunoglobulin for intravenous administration (IVIg)
Replaces: CPMP/BPWG/388/95 Rev 1 (Adopted by TGA 19 April 2001)
Effective: 1 June 2014
6. Cardiovascular system

Hình 7
7. Hypertension
How to access a pdf document
EMA/CHMP/29947/2013/Rev. 4 (pdf,212kb) (link is external)
Guideline on clinical investigation of medicinal products in the treatment of hypertension
Replaces: EMA/238/1995/Rev. 3 Guideline on clinical investigation of medicinal products in the treatment of hypertension (Adopted by TGA 17 August 2015)
Effective: 6 January 2017
EMA/CHMP/50549/2015 (pdf,114kb) (link is external)
Reflection paper on assessment of cardiovascular safety profile of medicinal products
Published for information January 2017
EMA/CHMP/206815/2013 (pdf,191kb) (link is external)
Paediatric addendum to the note for guidance on the clinical investigation on medicinal products in the treatment of hypertension
In place of: EMEA/CHMP/EWP/545456/2008 Concept Paper on the Need for the Development of a Paediatric Addendum to the Note for Guidance on the Clinical Investigation on Medicinal Products in the Treatment of Hypertension (Provided for information 1 May 2009)
Effective: September 2015
8. Lipid disorders
EMA/CHMP/748108/2013, Rev. 3 (pdf,204kb) (link is external)
Guideline on clinical investigation of medicinal products in the treatment of lipid disorders
Replaces: EMA/CHMP/748108/2013 Guideline on clinical investigation of medicinal products in the treatment of lipid disorder (Adopted by TGA 1 August 2014)
Effective: 6 January 2017
EMA/CHMP/494506/2012 (pdf,124kb) (link is external) (EMEA/CHMP/EWP/213057/2010)
Paediatric addendum to CHMP guideline on clinical investigation of medicinal products in the treatment of lipid disorders
In place of: EMEA/CHMP/EWP/350495/2009 Concept paper (provided for information 26 March 2010)
Effective: 1 August 2014
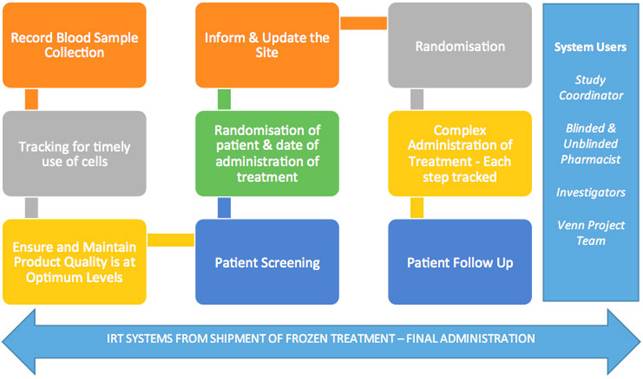
Hình 8
9. Pulmonary arterial hypertension
EMEA/CHMP/EWP/356954/2008 (pdf,62kb) (link is external)
Guideline on the Clinical Investigations of Medicinal Products for the Treatment of Pulmonary Arterial Hypertension
Effective: 28 May 2010
EMA/CHMP/213972/2010 (pdf,105kb) (link is external)
Paediatric Addendum to the CHMP Guideline on the Clinical Investigations of Medicinal Products for the Treatment of Pulmonary Arterial Hypertension
In place of: EMEA/CHMP/EWP/644261/2008 concept paper on this topic (provided for information 1 May 2009)
Effective: 1 August 2014

Hình 9
10. Arrythmias
EMA/CHMP/341363/2014 (pdf,264kb) (link is external)
Guideline on clinical investigation of medicinal products for prevention of stroke and systemic embolic events in patients with non-valvular atrial fibrillation
Effective: 26 December 2014
TGA annotation:
·Section 8.1:
·The collection of plasma/serum samples for the measurement of drug level and/or anticoagulant effect at the time of outcome events (e.g. bleeding, SEE, stroke) is recommended.
·The dossier must include specific discussion regarding non-specific and specific reversal agents and/or antidotes.
·The dossier must include a discussion regarding the utility of routine and/or ad hoc laboratory monitoring (drug levels or measures of anticoagulant effect) for the safe and efficacious use of the product. The availability of the laboratory tests mentioned should be included in the discussion.
·Section 6.2 (3):
·Where a study includes outcomes from 'VKA-naïve patients' meeting the definition provided in this guidance, a sensitivity analysis must be conducted with results from patients with no previous VKA exposure.
CHMP/ICH/2/04 (pdf,219kb) (link is external)
ICH Topic E 14
Note for Guidance on Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs
Effective: 9 February 2006
TGA annotation:
QT prolongation would be of regulatory concern if either the estimated QT prolongation was >5ms OR the upper bound of the 95% confidence interval was >10ms

Hình 10
EMA/CHMP/ICH/310133/2008 (pdf,176kb) (link is external)
ICH guideline E14 - questions and answers [on CHMP/ICH/2/04. The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs]
Effective: 15 September 2014
CPMP/EWP/237/95 (pdf,73kb) (link is external)
Note for guidance on antiarrhythmics
Effective: August 1997
EMA/CHMP/EWP/213056/2010 (pdf,177kb) (link is external)
Addendum to the Guideline on antiarrhythmics on atrial fibrillation and atrial flutter [CPMP/EWP/237/95]
Effective: 15 September 2014
10. Venous thromboembolism
How to access a pdf document
EMA/CHMP/325170/2012 (former CPMP/EWP/707/98 Rev. 1 corr) (pdf,227kb) (link is external)
Guideline on clinical investigation of medicinal products for prevention of venous thromboembolism (VTE) in patients undergoing high VTE-risk surgery
Replaces: CPMP/EWP/707/98 Rev 1 Guideline on Clinical Investigation of Medicinal Products for Prophylaxis of High Intra- and Post-Operative Venous Thromboembolic Risk (Adopted by TGA 8 December 2008)
Effective: 1 August 2014
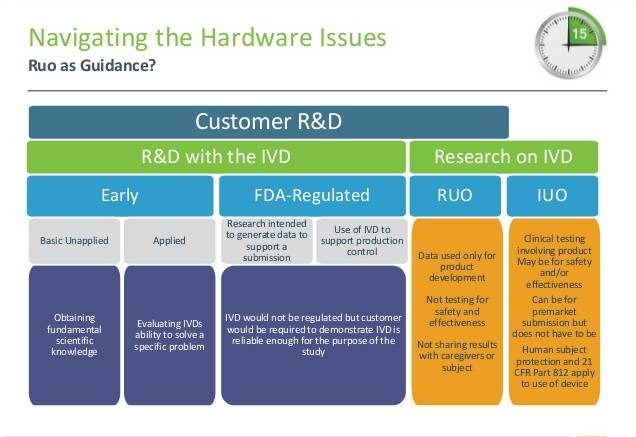
Hình 11
CPMP/EWP/6235/04 (pdf68,kb) (link is external)
Guideline on Clinical Investigation of Medicinal Products for the Prophylaxis of Venous Thromboembolic Risk in Non-Surgical Patients
Effective: 29 September 2006
EMA/CHMP/41230/2015 (pdf,225kb) (link is external)
Guideline on clinical investigation of medicinal products for the treatment of venous thromboembolic disease
Replaces: CPMP/EWP/563/98 Note for Guidance on Clinical Investigation of Medicinal Products for the Treatment of Venous Thromboembolic Disease (Adopted by TGA 25 January 2001)
Effective: 10 November 2016